Honokiol as an Effective Antimicrobial Compound against Causative Agent of American foulbrood, Paenibacillus larvae
Abstract
Recently, number of honeybees (Apis mellifera) has visibly decreased because they are vulnerable to some diseases like American foulbrood disease. American foulbrood disease, which is caused by Paenibacillus larvae, is emerged as great cause of decrease in number of honeybees. After antibiotic-resistant strain emerged, it is now more difficult to treat those pathogens successfully. Researches on finding alternative antibacterial compound are ongoing. In this study, we examined the antibacterial effect of honokiol on P. larvae. Honokiol showed great antibacterial effect with minimum inhibitory concentration of 12.5 μg/mL and minimum bactericidal concentration of 50 μg/mL. An agar diffusion test also confirmed the anti-Paenibacillus larvae activity of honokiol with an inhibitory zone of 9±0.5 mm. Since honokiol is known to interact membrane of some bacteria, we measured 260 nm absorbing particles, which could be induced by leakage of cells, and confirmed that the leakage of P. larvae occurred in dose-dependent manners. However, result of crystal violet assay suggested that honokiol has only mild anti-biofilm formation effect on P. larvae, which means honokiol controls the bacteria by inducing the bursting of membrane. Finally, an additive effect of honokiol with tetracycline and terramycin was found using a checkerboard assay with a fractional inhibitory concentration index value of 0.5.
Keywords:
Paenibacillus larvae, Honokiol, Antibacterial agent, Membrane leakageINTRODUCTION
Honeybees (Apis mellifera) are indispensable in our ecosystem and some important industries. Their role as pollinators help many plants to prosper which made them value over $14.6 billion in crop and fruit industry (Morse and Calderone, 2000). However, due to diseases like American foulbrood (AFB) the population of honeybees has been steadily decreasing and caused many problems.
Paenibacillus larvae is a causative agent of AFB (White, 1906). Since it can be easily spread over entire colony and could effect on larva of honeybees, it has been a great problem for honeybees. AFB has now been spread all over the world and appeared frequently (Genersch et al., 2006). Even though terramycin has been used to treat P. larvae (Gochnauer, 1951), unfortunately, P. larvae resistant to terramycin has arose over the world (Miyagi et al., 2000).
So far, many studies have been conducted to reduce the population of Paenibacillus larvae. However, it had many difficulties because P. larvae is resistant to heat and various chemicals. Therefore, it is urgent to find new antimicrobial substances, which can kill them effectively, rather than stopping the growth of P. larvae.
Magnolia obovata, which is a tree used as stomachic herb in Korea, Japan and China (Huang, 1998). Honokiol is a compound, which is known to have a great antifungal activity and can be easily found in the bark of M. obovata (Bang et al., 2000). It is also known as great anxiolytic agent (Maruyama et al., 1998).
The present study provided an antibacterial activity of honokiol and proof that it could serve as an effective inducer of bursting of P. larvae. This study also showed an additive effect of honokiol with well-known antibiotics, terramycin and tetracycline and we strongly suggested that honokiol could be used as a novel chemical with anti-P. larvae activity.
MATERIALS AND METHODS
Microorganism strain and growth conditions
P. larvae (strain ATCC 9545) were developed in Mueller-Hinton broth, yeast extract, potassium phosphate, glucose and sodium pyruvate (MYPGP broth) at 37℃ with shaking overnight (Dingman and Stahly, 1983). Before use, cells were centrifuged at 3000 rpm for 10 minutes. After old media was replaced with new media, cells were incubated again for 2 hours before use.
Compound preparation
All compounds used in this study, honokiol (CAS: 35354-74-6, Chemfaces), terramycin (CAS: 2058-46-0, Tokyo Chemical Industry) and tetracycline (CAS: 60-54-8, Sigma-Aldrich), were dissolved in dimethyl sulfoxide (DMSO) to 10 mg/mL before use.
Investigation of antimicrobial effects of compounds
Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) were determined prior to other experiments. To determine MIC, a two-fold micro-dilution method was used as described in previous studies (Zgoda and Porter, 2001). 105 CFU/mL of P. larvae were added into each well of a 96 well plate and the compound in each lane were then diluted two-fold from 100 μg/mL to 0.156 μg/mL. After a 48-hour incubation at 37℃, the minimum inhibitory concentration of each compound was measured.
To determine MBC, broth from clear wells after MIC test were spread on MYPGP agar plate. Plates were placed in 37℃ for another 48 hours and Minimum bactericidal concentration of each compound was measured.
Disk diffusion test
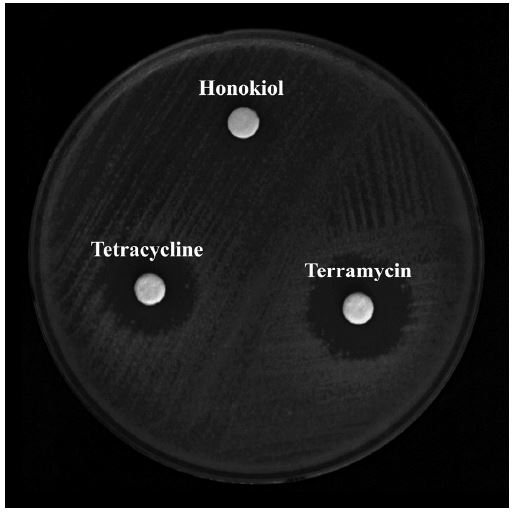
Antibacterial sensitivity of compounds was determined using the agar diffusion method (Fiebelkorn et al., 2003). P. larvae was diluted to an optical density of 0.1 at 600 nm and they were spread onto an MYPGP agar plate. 50 μg of each compound was added to a 0.3 mm-radius paper disk and placed on the MTPGP agar plate. Cells were incubated for 24 hours at 37℃ and diameter of the clear zone was measured.
Checking leakage with 260 nm absorbing particles
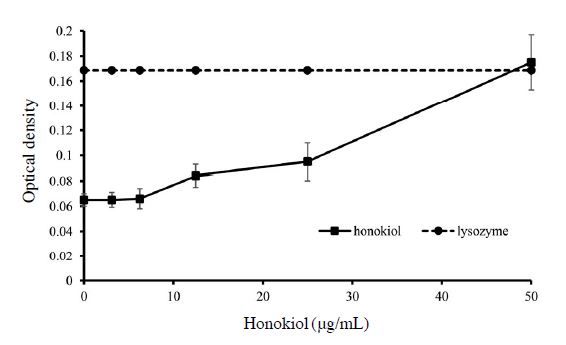
In order to verify the leakage of cells after treatment of the compound, 260 nm absorbing particles were measured. To stop further proliferation, cells were incubated in PBS for 2 hours. Compounds were then treated with concentration ranging from 3.12 to 50 μg/mL for 1 hour. Lysozyme as a positive control was treated to determine maximum optical density after bursting of the cells, each concentration of the compound was measured without cells. Lysate released after leakage was measured by microplate spectrophotometer (Epoch, USA) at an optical density of 260 nm. All values were revised by deducting the value of corresponding negative control (Chen and Cooper, 2002; Junaidah et al., 2014).
Visualizing the bursting with transmission electron microscopy
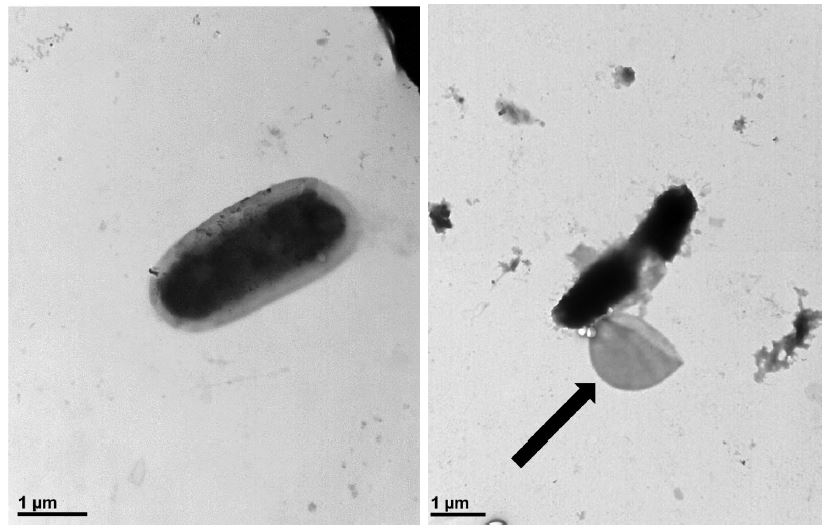
Transmission electron microscopy (TEM) visualized bursting P. larvae cells after treatment of honokiol on bacterial cells. The assay was performed using an 80 kV transmission electron microscope (JEM1010, JEOL) at NICEM (Seoul national university, South Korea). One hour before TEM imaging, honokiol treatment was carried out at a concentration of 6.25 μg/mL. Samples were loaded onto a grid and dyed with phosphotungstic acid (PTA). The grid was washed to remove any unattached cells. Cells that had not been treated with honokiol were used as a control.
Crystal violet assay
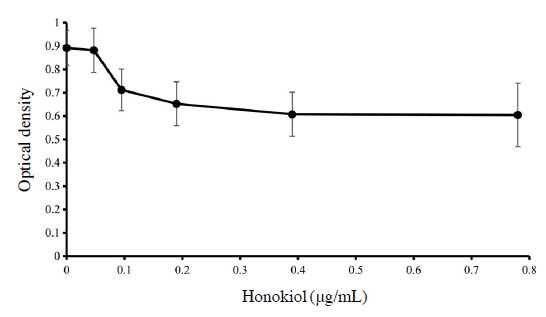
A microtiter dish biofilm formation assay (O’Toole, 2011) was performed to determine inhibition of biofilm formation of P. larvae by honokiol. 100 μL of 105 CFU/mL of P. larvae was added to each well of a 96 well-plate. Honokiol was treated with the concentration from 0.047 to 6.25 μg/mL, the plate was placed at 37℃ for 24 hours. After incubation, the plate was rinsed with distilled water and then stained with 0.1% crystal violet. Leftover crystal violet solution was removed after 20 minutes. Elution was done with 30% acetic acid and optical density of eluted solution was measured in new plate at 550 nm.
Checkerboard assay
A checkerboard assay was performed to check for synergy effects between honokiol and two well-known antibiotics, tetracycline and terramycin (Schwalbe, 2007). All cells were treated with honokiol with tetracycline or terramycin with different concentration combinations. Honokiol was diluted horizontally as two-fold, while antibiotics were treated vertically by two-fold dilution. FIC index values were measured. The combination is considered synergistic when the FIC value is under 0.5, indifferent when the FIC value is in range between 0.5 to 2, and antagonistic when the FIC value is over 2.
RESULTS
Investigation of antimicrobial effect of compounds
Honokiol had great MIC value (12.5 μg/mL) compared to those of terramycin (25 μg/mL) and tetracycline (25 μg/mL) (Table 1). However, MBC value did not show much difference among the compounds (Table 2). They all had same MBC value of 50 μg/mL.

Minimum inhibitory concentration (MIC) values (μg/mL) of honokiol, terramycin and tetracycline against P. larvae after 48 h treatment
Disk diffusion test
Honokiol showed mild antibacterial activity against P. larvae (Table 3 and Fig. 1) with inhibitory zones of 9±0.5 diameters at 24 hours and 36 hours. Although it did not show better activity than terramycin (17±1 at 24 hours and 16±0.5 at 36 hours) and tetracycline (16±1 at 24 hours and 15±1 at 36 hours), it is definitely considerable as an antibacterial agent since it had great MIC value.

Antimicrobial susceptibility test results based on agar diffusion analysis shown with diameter of the cleared area
Checking leakage with 260 nm absorbing particles
The release of 260 nm absorbing materials increased after treatment of honokiol (Fig. 2). When concentration of honokiol was lower than MIC value (12.5 μg/mL), it only had some subtle changes. However, at the concentration of 12.5 μg/mL, 260 nm absorbing particles significantly leaked out from membrane (0.084±0.005) and at the concentration of 50 μg/mL, the optical density of 260 nm absorbing particles reached the same as optical density of lysozyme-treated samples (0.175±0.022 and 0.168 respectively).
Visualizing the bursting with transmission electron microscopy
Although there clearly was a leakage of P. larvae after honokiol treatment, it was not certain whether it induced the bursting of cells or it made only some crack on the membrane. When it was visualized by TEM, honokiol surely induced a bursting of the cells (Fig. 3). Unlike control cells, honokiol-treated cells had their membrane destroyed and had their inner-membrane left out.
Crystal violet assay
Since biofilm is so crucial to bacteria survival, biofilm disturbance of honokiol was examined using crystal violet assay (Fig. 4). There was no big difference between biofilm of P. larvae without honokiol and with 0.047 μg/mL of honokiol. When the concentration was higher than 0.047 μg/mL, biofilm formation kept decreasing as the concentration of honokiol increased. All of the results appeared in dose-dependent manner.
Checkerboard assay
Synergy of honokiol with two antibiotics, terramycin and tetracycline, was determined using checkerboard assay (Table 4). When they were treated individually, the MIC value was 12.5 μg/mL, 25 μg/mL and 25 μg/mL respectively. However, when antibiotics were treated together with honokiol, MIC value has droped to 3.12 μg/mL, 6.25 μg/mL and 6.25 μg/mL respectively. Both checkerboard assay of honokiol & terramycin and honokiol & tetracylcline had FIC value of 0.5. According to previous studies (Schwalbe, 2007), It can be considered as additive compound of two antibiotics.
DISCUSSION
The use of honokiol has been studied all around east Asia where M. obovata can be easily found. It is considered as a medicine for stomachache for a long period of time (Huang, 1998). Recently, its variety of effects has been discovered. Its anxiolytic activity was revealed (Maruyama et al., 1998), and its antiangiogenic and antitumor activity has also reviewed (Fried and Arbiser, 2009). In this study, we have confirmed that honokiol can be an effective antibacterial agent against P. larvae.
Our results established that honokiol control the growth of P. larvae effectively. It is worth to note that honokiol had greater antibacterial activity against P. larvae than terramycin and tetracycline with half of MIC value of those two antibiotics. To use honokiol as a chemical to control the growth of P. larvae, we might expect it is used as liquid solution and sprayed directly to the bacteria. When honokiol was treated with terramycin and tetracycline, the use of chemical was lessened. Like explained above, honokiol could be great antibacterial compound because it may slow or even stop the rise of P. larvae with antibiotics resistance.
Our results also demonstrated the target of honokiol against P. larvae. We showed that honokiol ruptures the membrane, resulting the bursting of the cell and it could be the reason why honokiol had low MIC value. From the images of TEM, it was curious how honokiol was so effective even it was treated only for an hour. It greatly induced the bursting of cells fast, proving its time-effective antimicrobiol activity. From the studies done before, we could predict the ability of honokiol to rupture the membrane of P. larvae comes from its structure (Woodbury et al., 2013). Since honokiol is classified as polyphenol family, it could easily merge with cell membrane proteins by interactions between molecules like hydrogen bonding or hydrophobic interactions. Identifying the exact mechanism of honokiol interacting membrane needs to be identified in further study. Its ability to interrupt biofilm formation were surprisingly effective considering the amount of honokiol used for the crystal violet assay. Considering that honokiol already had its ability interrupt biofilm formation at concentrations only over 0.047 μg/mL, it is now confirmed to be a considerably efficient anti-biofilm agent.
The safety of honokiol was not demonstrated in this study. In view of commercial use of this compound, its cytotoxicity will be measured and in vivo experiments using honeybees will be performed in further study.
Acknowledgments
This research was supported by the IPET (116094-03-1-SB010).
FOOTNOTES
Financial Disclosure: This research was conducted in the absence of any commercial or financial relationships that could be construed as posing potential conflicts of interest.
References
-
Bang, K. H., Y. K. Kim, B. S. Min, M. K. Na, Y. H. Rhee, J. P. Lee, and K. H. Bae, (2000), Antifungal activity of magnolol and honokiol, Arch. Pharm. Res., 23(1), p46-49.
[https://doi.org/10.1007/bf02976465]

-
Chen, C. Z., and S. L. Cooper, (2002), Interactions between dendrimer biocides and bacterial membranes, Biomaterials, 23(16), p3359-3368.
[https://doi.org/10.1016/s0142-9612(02)00036-4]

- Dingman, D. W., and D. P. Stahly, (1983), Medium promoting sporulation of Bacillus larvae and metabolism of medium components, Appl. Environ. Microbiol., 46(4), p860-869.
-
Fiebelkorn, K. R., S. A. Crawford, M. L. McElmeel, and J. H. Jorgensen, (2003), Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci, J. Clin. Microbiol., 41(10), p4740-4744.
[https://doi.org/10.1128/jcm.41.10.4740-4744.2003]

-
Fried, L. E., and J. L. Arbiser, (2009), Honokiol, a multifunctional antiangiogenic and antitumor agent, Antioxid. Redox Signal., 11(5), p1139-1148.
[https://doi.org/10.1089/ars.2009.2440]

-
Genersch, E., E. Forsgren, J. Pentikäinen, A. Ashiralieva, S. Rauch, J. Kilwinski, and I. Fries, (2006), Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation, Int. J. Syst. Evol. Microbiol., 56(3), p501-511.
[https://doi.org/10.1099/ijs.0.63928-0]

- Gochnauer, T. A., (1951), Drugs fight foulbrood disease in bees, Minn. Home Fm. Sci., 9, p15.
- Huang, K. C., (1999), The Pharmacology of Chinese Herbs, 2nd ed., CRC Press.
- Junaidah, A. S., H. H. Lian, D. F. Basri, and N. M. Zin, (2014), Mode of action of endophytic Streptomyces sp., SUK 25 extracts against MRSA; microscopic, biochemical and time-kill analysis, Int. J. Pharm. Sci. Rev. Res., 30(1), p11-17.
-
Maruyama, Y., H. Kuribara, M. Morita, M. Yuzurihara, and S. T. Weintraub, (1998), Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine, J. Nat. Prod., 61(1), p135-138.
[https://doi.org/10.1021/np9702446]

-
Miyagi, T., C. Y. Peng, R. Y. Chuang, E. C. Mussen, and M. S. Spivak, (2000), Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States, J. Invertebr. Pathol., 75(1), p95-96.
[https://doi.org/10.1006/jipa.1999.4888]

- Morse, R. A., and N. W. Calderone, (2000), The value of honey bees as pollinators of US crops in 2000, Bee Culture, 128(3), p1-15.
- O’Toole, G. A., (2011), Microtiter dish biofilm formation assay, J Vis Exp., 47, p2437.
-
Schwalbe, R., L. Steele-Moore, and A. C. Goodwin, (2007), Antimicrobial Susceptibility Testing Protocols, CRC Press.
[https://doi.org/10.1201/9781420014495]

- Viksø-Nielsen, A., T. M. Christensen, M. Bojko, and J. Marcussen, (1997), Purification and characterization of β-amylase from leaves of potato (Solanum tuberosum), Physiol. Plant., 99(1), p190-196.
- White, G. F., (1906), Bacteria of the Apiary, Technical series (United States. Bureau of Entomology), no. 14.
-
Woodbury, A., S. P. Yu, L. Wei, and P. García, (2013), Neuro-modulating effects of honokiol: a review, Front. Neurol., 4, p130.
[https://doi.org/10.3389/fneur.2013.00130]

-
Zgoda, J. R., and J. R. Porter, (2001), A convenient microdilution method for screening natural products against bacteria and fungi, Pharm. Biol., 39(3), p221-225.
[https://doi.org/10.1076/phbi.39.3.221.5934]